ANNEX II. Phenomenology of ozone concentrations
For a better understanding of the report, some of the main
characteristics of ambient ozone are summarized here. For more advanced information on
ozone and its photochemical formation the reader is referred to information documents
provided by DGXI (Borrell and van den Hout, 1995; Derwent and van den Hout,1995) and to
reports prepared in the framework of UN-ECE Convention on long-range transport of air
pollution (Malik et al., 1996; Hjellbrekke, 1996; Barrett and Berge, 1996) and
EUROTRAC (see e.g. Borrel et al.,1997; Hov, 1997).
Ozone is a secondary air pollutant formed in the atmosphere under the
influence of sunlight. Ozone formation occurs at all levels in the atmosphere, from ground
level up to the stratosphere. Here the discussion is limited to ozone at ground level. It
has been shown that under the present conditions in Europe the ozone exposure of humans,
vegetation and materials leads to adverse effects.
The precursors of the ozone formation are Volatile Organic Compounds
(VOC), carbon monoxide (CO) and nitrogen oxides (NOx). VOC and CO act as
"fuels" as they are oxidized in the process; the nitrogen oxides play an
important role as "catalysts": they are not 'consumed' in the formation process
but are essential for the continuation of the process. However, nitrogen oxides are
consumed in side reactions by which they are further oxidized to nitric acid or nitrates.
For the continuation of the photochemical oxidation process a continuous injection of
nitrogen oxides is therefore necessary.
The ozone formation takes place on various time and spatial scales: on
the local scale as in urban areas as Athens or Milan, on the regional scale as is
demonstrated by the photochemical episodes in Central and Northwest Europe and on the
hemispheric/global scale. Highly reactive VOCs are important precursors on the local and
regional scale whereas the less reactive, relatively long-lived VOCs such as methane
contribute to ozone formation on the global/hemispheric scale.
The role of nitrogen oxides is complex and may be different at various
distances from the source. In heavily populated areas the ozone concentrations may be
lower than the regional concentrations due to chemical scavenging by local nitrogen oxide
emissions. This scavenging is presented by the chemical reaction:
O3 + NO ® NO2 + O2
(R1)
Nitrogen dioxide (NO2) formed in this reaction can be seen as
'potential ozone' as in the photolysis of NO2, nitrogen monoxide, NO, and ozone
are produced:
NO2 (+ O2) ® O3
+ NO (R2)
As both the time scales of the NO-scavenging reaction (R1) and of the NO2
photolysis are relatively short, an equilibrium between the three components will be
established:
O3 + NO « NO2 + O2
(E1)
Note that the sum of O3 and NO2 (frequently
indicated as oxidant or Ox) is independent of the equilibrium. Knowledge on
simultaneously measured NOx concentrations in general and NO2
concentrations in particular is therefore important for interpretation of ozone levels.
The oxidant levels are spatially less variable than the ozone levels. A mapping procedure
based on oxidant levels is therefore preferred.
The time scales of photochemical ozone formation are generally longer
than the time scale associated with the above reaction R1 and R2 and close to NOx
sources a decrease in ozone concentrations may be observed; at larger distances to the
source the ozone levels will increase again. In Figure II.1 the ozone concentration in an
air parcel passing a NOx source is schematically presented.
One of the consequences of this interaction with NOx is that
the representativeness of ozone monitoring stations depends strongly to what extent the
station is influenced by local NOx sources: concentrations measured at a
station in a traffic situation will be representative for its direct surroundings only
(e.g. less than a few 100 m) whereas a background station may measure concentrations
representative for an area of several tens of kilometres. The information requested in
Article 4 of the Directive should form the basis on which a representative area for each
of the monitoring stations could be defined.
At ground level ozone concentrations generally show a strong diurnal
variation. At night concentrations are low, both caused by removal by dry deposition and
by titration by NO-emissions according to reaction R1. In the morning concentrations
increase, caused not only by the sunlight induced photochemical formation but also by the
downward mixing of higher, ozone-rich layers. In the afternoon both processes will become
less important and concentrations will decrease again when the loss processes dominate.
The maximum concentration is frequently found in the late afternoon, around 16.00. For the
eight hour periods reported in the framework of the Ozone Directive, the highest value
will generally be observed between 12.00 and 20.00.
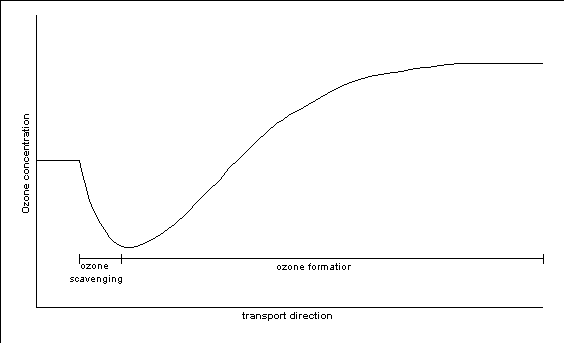
Figure II.1 Schematic presentation of ozone concentrations downwind
of a large NOx source where ozone scavenging will take place.
References
Borrell, P. and van den Hout D. (1995) Tropospheric ozone - a review of
current understanding. Doc.Ref XI/363/95, European Commission, Brussels.
Borrell P., Builtjes P., Grennfelt P. and Hov Ø. (eds.) (1997)
Photo-oxidants, acidification and tools. Policy application of EUROTRAC results.
Springer-Verlag, Berlin.
Barrett K. and Berge E. (eds.) (1996) Transboundary air pollution in
Europe. Part 1: Estimated dispersion of acidifying agents and of near surface ozone.
EMEP/MSC-W Report 1/96. The Norwegian Meteorological Institute, Oslo, Norway.
Derwent, D. and van den Hout D. (1995) Computer modelling of ozone
formation in Europe. Doc.Ref XI/364/95, European Commission, Brussels.
Hjellbrekke A.G.(1996) Ozone Measurements 1993-1994, EMEP/CCC report
1/96, NILU, Kjeller, Norway.
Hov Ø. (Editor) (1997) Tropospheric Ozone Research, Springer Verlag,
Berlin.
Malik S., Simpson D., Hjelbrekke A. G., ApSimon H. (1996) Photochemical
model calcu-lations over Europe for summer 1990. Model results and comparison with
observation. EMEP/MSC-W report 2/96, The Norwegian Meteorological Institute, Oslo, Norway.


Document Actions
Share with others