2. Tropospheric ozone: background information
2.1 The issue of ozone in Europe
Photochemical pollution is formed from emissions of nitrogen oxides (NOx, where NOx = NO + NO2) and of volatile organic compounds (VOCs) and carbon monoxide (CO) in the presence of sunlight. Ozone (O3), the major photochemical pollutant, is transported across national boundaries (Simpson and Malik, 1996). Emissions of NOx are responsible for much of the ozone formation occurring in rural areas. In more densely populated regions, in particular close to cities, ozone formation is enhanced by VOC emissions. VOCs are mainly released from road traffic and the use of products containing organic solvents. NOx and CO are mostly emitted from transport and combustion processes. After release, these precursors are dispersed by wind and atmospheric turbulence. The freshly emitted pollutants mix with other pollutants, including ozone, present in background air, and a complicated process of chemical reactions and continuous dilution takes place.
Exposure to ozone induces effects on health and the environment,causing respiratory difficulties in sensitive people and possible damage to vegetation and ecosystems (WHO, 1996a, b). Significant responses in both humans and plants occur at or close to current ambient concentrations of ozone (UN-ECE, 1996). Threshold values set for the protection of human health, vegetation and ecosystems are exceeded frequently in most European countries and therefore widespread adverse effects on the European population, crops and natural vegetation may be expected (de Leeuw and van Zantvoort, 1996; Hjellbrekke et al., 1996). Ozone in the troposphere is also of relevance to the climate change issue since ozone is a greenhouse gas. It is currently estimated that tropospheric ozone adds 0.4 W.m-2 to the current enhanced climate forcing of 2.45 W.m-2. The total forcing is a result of the increase in long lived compounds only (CO2, CH4, N2O, halocarbons) (IPCC, 1995). The issue of formation, effects and abatement of ozone is complex, and due to its transboundary nature, an international effort to develop a coherent policy will be necessary to cope with the problem (Amann et al., 1996).
2.2 Chemical formation of ozone
The photochemical chain reaction which produces ozone is initiated and maintained by reactive radicals. In the process, other products are formed such as peroxy acetyl nitrate, nitric acid, aldehydes, organic acids, particulates and many short-lived radical species. VOCs act as "fuel" in the ozone formation process, whereas NO functions more or less as a catalyst, since it is regenerated in the formation process. NO also plays a key role in the regeneration of the reactive radicals and the further progress of the reactions. The simplified photochemistry of ozone, which is in reality a complex and highly non-linear process, is shown in Box 1.
High concentrations of freshly emitted NO locally scavenge O3, a process leading to formation of NO2. Close to the sources this titration process can be considered as an ozone sink. In addition, high NO2 concentrations deflect the initial oxidation step of VOCs by forming other products (e.g. nitric acid), which prevents the net formation of O3. Because of these reactions, a decrease in NOx can lead to an increase in O3 at low VOC/NOx ratios, as is the case in cities. In this often called VOC-limited regime, emission control of organic compounds is more efficient to reduce peak values of ozone pollution locally.
As an air mass moves away from an urban centre its VOC/NOx ratio changes due to further photochemical reactions, meteorological processes and the occurrence of fresh emissions. The concentration of NOx decreases faster than that of VOC and consequently the VOC/NOx ratio is amplified. At high VOC/NOx ratios occurring in background situations, the chemistry tends towards the NOx-limited case and NOx reductions are considered more effective to reduce ozone in these situations. Recent work (Kramp et al., 1994; Flocke et al., 1994) indicates that the photochemistry in urban plumes proceeds faster than was previously assumed. The oxidation of VOCs leads to more ozone over a shorter time period, and to a faster removal of NOx. Hence the regime where ozone formation is controlled by the concentration of NOx is reached more quickly than previously thought (Borrell et al., 1995).
Box 1: The photochemistry of ozone formation in simplified form
|
VOC + OH + O2 ® RO2 + H2O
RO2 + NO + O2 ® NO2 + HO2 + CARB
HO2 + NO ® NO2 + OH
2(NO2 + hv +O2 « NO + O3) |
|
net:
|
(NOx + OH +) VOC + 4O2 ® 2O3 + CARB + H2O (+ NOx + OH) |
| VOC stands for volatile organic compound and CARB for carbonyl compounds, which play the role of hydrocarbons in further oxidation steps. OH and HO2 are short-lived radicals which play an important role in the ozone formation process. |
The complexity of the effect of NOx emission reductions on O3 formation can be illustrated by the "week-end effect". Dumont (1996) documented that O3 levels in Belgian conurbations were found to be significantly higher in the week-end than during the week. During "smog" summers the average afternoon peak was about 20% higher on Saturdays and Sundays in comparison to the "working day" afternoon peak. The opposite pattern occurred for NO2; this species was much lower on Saturdays and Sundays. However, the sum of O3 and NO2, often called Ox, was similar no matter what day was taken. This can be explained by less conversion of O3 by NO into NO2 as a result of the low level of NOx emissions during the week-end in Belgian cities (about 30% lower). In this case, this local ozone depletion effect is more important than the reduction of ozone from reduced precursor (NOx and VOC) emissions. Further ozone variations over weekdays suggest additional non-local contributions. In Austria, at stations in or near Vienna, average maximum ozone peak concentrations also show a distinct weekly pattern, however with lowest levels on Mondays and Tuesdays. This is believed to reflect ozone reduction from reduced precursor emission in a larger area, and the effect of transport (Schneider,1998). Brönniman and Neu (1997) conclude from analysis of Swiss data that there are two distinct patterns in the weekly cycle of ozone. When the meteorology is not favourable to ozone production higher concentrations were observed during the week-end. However, during favourable conditions the mean ozone peak on Sundays was 10-15% lower than on workdays.
It is worth noting that it is only as a result of initial and small NOx reductions, in the absence of concurrent reductions in VOC emissions, that the counter productive "week-end effect" may occur. To attain acceptable ozone levels, and to pass the initial counter-productivity threshold, abatement of a major fraction of both NOx and VOC emissions is necessary.
2.3 Spatial scales
Formation of ozone occurs over various scales, from local, as in urban areas such as Athens and Milan, through regional, as in central and northwest Europe, to hemispheric, as in the increase in background concentrations over northern mid-latitudes. Locally formed oxidants generally show large temporal and spatial variations with high peak concentrations caused by emissions mainly occurring during the same day. Regional oxidant formation most often occurs in connection with stable high pressures and high concentrations may remain for a number of days (Cox et al, 1977; Guicherit and van Dop, 1975; Grennfelt and Schjoldager, 1984; Borrell et al., 1995). The balance between (local) formation and (long-range) transport in the ozone climatology in a particular area determines the effectiveness of the range of local to pan-European emission reductions.
2.3.1 The phenomenology of rural ozone in Europe
Grennfelt et al. (1987; 1988) and Feister and Pedersen (1989) were the first to report summer ozone levels in Europe showing an increasing gradient from the the north-west to the south-east part of the OXIDATE network. Unfortunately, their analysis did not extend much south of the Alps due to the limited availability of data from the south and east of Europe. Later reports (NILU/CCC 1990 ~1996) confirmed the gradient pattern in ozone.
A quantitative estimate of ozone in summer and winter was provided by Beck and Grennfelt (1994). Based on measurements from 68 rural and background stations they found that the average diurnal maximum in summer ranged from 60-80 μg.m-3 in the north-western part to 120-140 μg.m-3 in the central part of Europe. Figure 1 illustrates a modelled version of the gradient in the average diurnal maximum ozone concentration during summer over Europe (Simpson et al., 1997). The European marine boundary layer background concentration, i.e. the concentration in air advected from the Atlantic, was established at 60-65 μg.m-3 (Borrell et al., 1995). It may be useful to note that the information reported in the framework of the Directive does not allow the assembly of this general picture for rural ozone. Measured data from the Eurotrac-TOR and EMEP networks and EMEP model activities were used. It is also to be noted, that the spatial distribution of ozone over Europe varies considerably from year to year, and depends on the statistic considered (compare Figure 1 for summer daily maximum to Figure 16 for AOT60).
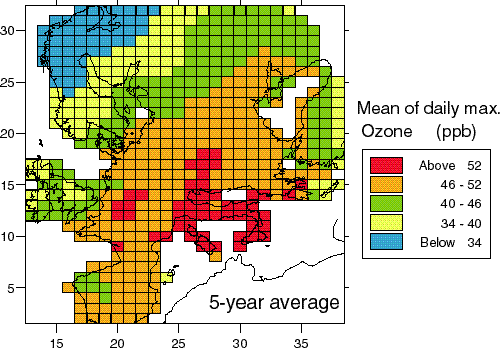
Figure 1: The modelled 5-year mean of the daily summer maximum concentration of ozone. The calculation was performed using constant emissions at the 1990 level and meteorology from 5 summers (1989, 1990, 1992, 1993 and 1994). 1 ppb O3 » 2 μg.m-3. Source: Simpson et al., 1997
The seasonal variation of ozone, with a broad summer maximum and a winter minimum, is observed at many individual sites on the continent. On a seasonal basis, atmospheric processes in the polluted European boundary layer add 30-40% to the boundary layer background concentration in summer. Most rural inland stations show a typical diurnal pattern during the summer months, with a minimum in the morning and a maximum in the afternoon. The decrease during the night and early morning is caused by dry deposition. At stations closely influenced by emissions, the effect reflects the titration from NO as well. After sunrise the photochemical formation from precursors starts and gradually grows as the amount of sunlight increases. The mixing of air from layers aloft and from the free troposphere also plays a role. Unpolluted coastal and high-elevation stations often show a less pronounced diurnal variation due to the small influence of dry deposition, and advection of homogeneous and relatively undisturbed air.
2.3.2 The phenomenology of episodes
Episodes of increased ozone occur over most parts of Europe every summer. During these episodes, many of which last for several consecutive days, ozone concentrations rise to several times the boundary layer background over large areas of Europe. The phenomenon of episodes usually occurs under anticyclonic conditions coinciding with increased sunlight, high temperatures and low wind speed. They are observed in both polluted urban areas and in less polluted rural regions. In the urban and suburban situation high concentrations are mainly due to photochemical production from precursors that are mostly emitted within the area. On the continental scale enhanced concentrations are the consequence of both in situ chemistry and transport from other regions. Bouscaren (1991), however, concluded that in the south of Europe photochemical smog is often of a local character.
Generally, close to the sources in city centres ozone concentrations are lower than those in suburbs and rural areas, mainly as a result of ozone scavenging by nitric oxide from traffic. During episodes, ozone levels can be considerably elevated in the suburbs and further downwind of the urban source areas. The build-up of ozone due to photochemical reactions takes several hours, and as a result, the highest levels of photo-oxidants can be expected some distance downwind of the sources. Lin et al. (1995) and Lindsay and Chameides (1988) found that the ozone concentration in an urban plume was easily twice the background level.
Several cities, in particular those in southern Europe, experience peak ozone levels in their urban centres. This occurs often as a result of stagnant air or sea breeze conditions in summertime anticyclonic situations. In the Mediterranean, large scale circulation cells are established and coastal emissions can be trapped for several days in the land-sea breeze. The worst photochemical oxidant episodes in the Mediterranean are probably linked to land-sea breeze circulation systems, affecting in particular the regions with major cities such as Barcelona, Marseille, Rome and Athens (Borrell et al., 1995). Case studies on these phenomena are reported for Athens, Valencia and Lisbon by Moussiopoulos (1994), Millán (1993) and Borrego et al., (1994). A short discussion on ozone in the Mediterranean is presented in Appendix 3.
2.4 Trends in tropospheric ozone
The first quantitative measurements of the O3 mixing ratio in Europe were made at the Observatoire de Montsouris near Paris, between 1876 and 1886. The 24-h average concentration was then about 20 μg.m-3 (Volz and Kley, 1988). It may be interesting to note that these data (1000 samples) show exceedances of the current EU threshold value for the protection of vegetation (65 μg.m-3 24-h average) during somewhat less than 1% of all observations. (Volz-Thomas, pers. comm.). Most of these occasions arose in February and one occurred in May and they reflect the influence of air with a free tropospheric origin reaching ground level.
In the 1950s the 24-h mean rural ozone levels had increased to 30 - 40 μg.m-3 and they continued to rise to 60 μg.m-3 in the 1980s (Feister and Warmbt, 1987). In the late 1990s daily mean concentrations are at least a factor of two higher than in the pre-industrial era (Borrell et al., 1995, Staehelin et al., 1994). Most of the O3 increase occurred in the 1970s associated with the tremendous growth in NOx emissions over that period. Over the last decade, no or little ozone increase has occurred generally over rural Europe. In the Netherlands concentrations decreased a little (Roemer, 1996) while in the south of Germany several sites report a 2% annual upward trend (Scheel et al., 1997). On the Irish coastal fringe of the Atlantic an upward trend of about 1% per year during summer due to polluted air from mainland Europe is observed (Simmonds, 1993).
Little work has been done on deriving trends in the occurrence of ozone episodes in Europe. However, some countries reported data on peak 98th percentile concentrations covering the period 1989 - 1996 in the framework of the ozone Directive (see Chapters 4 and 5). This data base indicates a significant upward trend in the 98th percentile of a few μg.m-3 at 2 sites in Belgium and Luxembourg whereas a significant downward trend over 1989 - 1996 is observed at 18 stations in the United Kingdom and the Netherlands. There was no evidence for any trend at 35 stations in these four countries (de Leeuw and van Zantvoort, 1997). No clear picture for a country or region emerged. A word of caution is necessary here. Co-located data on NOx were not available which prevents us from checking whether the observed trends are caused or masked by changes in NOx concentrations. We recommend to use the sum of NO2 and O3, often called Ox, as a parameter to overcome the influence of the titration effect because Ox is insensitive to titration (Guicherit, 1988). Furthermore, changes in techniques of measurements or operation procedures may bias the detection of trends (Roemer, 1997).
The occasional historical record, derived from semi-quantitative measurements only, is available for ozone in the urban environment. Annual ozone averages of 40 - 60 μg.m-3 were measured in Athens in the first two decades of this century (Cartalis and Varotsos, 1994). Similar levels in the 1890s were documented for Zagreb (Lisac and Grubisic, 1991).
The annual 98th percentile of ozone in Central London varied between 60 and 140 μg.m-3 and showed a significant trend of -2.8 μg.m-3 per year between 1973 and 1992 (PORG, 1987; Bower et al. 1991, 1994). Basic ozone statistics from several other north-west European urban stations show values in comparable ranges over the last 5 to 10 years. A record from a suburb station of Athens (Liosia) shows that the monthly mean concentration exhibited an average increase rate of about 15% per annum in the period 1984-1989. In 1987 the monthly mean values started to exceed 110 μg.m-3 (Moussiopoulos, 1994). Note that this value represents the current EU 8-h average threshold value for the protection of human health. In 1988 this threshold was exceeded on 140 days at this monitoring station.
The identification of a trend in ozone episodes in the urban environment may be more important. Table 3 presents the number of exceedances of the 110 μg.m-3 8-h average concentration (12-20 h) at several urban sites. The table shows that exceedances occur at all urban sites. Over the years available for this report, no significant trend can be detected. The year-on-year meteorological variation is likely to be the major cause of the large interannual variation.
Table 3: The number of exceedances of the 110 µg.m-3, 8-h average threshold value at a selection of (sub)urban sites in the period 1982 - 1995
| Country |
Station |
City |
1982 |
1983 |
1984 |
1985 |
1986 |
1987 |
1988 |
1989 |
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
| Belgium |
I.R.M. Av. Circulaire |
Bruxelles |
* |
* |
* |
* |
10 |
10 |
4 |
37 |
23 |
11 |
18 |
21 |
34 |
42 |
| Belgium |
St-Kruiswinkel |
Gent |
* |
* |
* |
10 |
8 |
14 |
7 |
16 |
33 |
7 |
21 |
9 |
20 |
21 |
| Belgium |
Namur Ville en Waret-Vezin |
Sites de fond |
* |
* |
* |
* |
6 |
10 |
12 |
39 |
41 |
0 |
11 |
9 |
31 |
32 |
| Greece |
Patission 147 |
Athens |
* |
0 |
0 |
* |
* |
10 |
17 |
17 |
14 |
1 |
* |
* |
* |
* |
| Greece |
Smyrni Cementery of N Smyrni |
Athens |
* |
* |
* |
* |
* |
20 |
48 |
60 |
34 |
71 |
* |
* |
* |
* |
| Greece |
Aspropyrgos |
Athens |
* |
23 |
9 |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
| Greece |
Pireas Platia Dimotikou Theatrou |
Athens |
* |
* |
* |
* |
* |
* |
59 |
83 |
30 |
8 |
* |
* |
* |
* |
| Netherlands |
Florapark |
Amsterdam |
* |
* |
* |
* |
7 |
9 |
8 |
27 |
30 |
6 |
19 |
9 |
14 |
20 |
| Netherlands |
Const.Rebecqueplein |
Den Haag |
* |
* |
* |
* |
0 |
11 |
8 |
28 |
37 |
17 |
23 |
9 |
20 |
19 |
| Netherlands |
Schiedamsevest |
Rotterdam |
* |
* |
* |
* |
8 |
9 |
7 |
32 |
20 |
11 |
20 |
* |
* |
* |
| Netherlands |
Kard. De Jongweg |
Utrecht |
* |
* |
* |
* |
* |
0 |
10 |
24 |
17 |
1 |
10 |
8 |
17 |
20 |
| Netherlands |
Witte Vrouwenstraat |
Utrecht |
* |
* |
* |
* |
* |
0 |
0 |
5 |
4 |
* |
4 |
* |
* |
* |
| Netherlands |
Tuin Utrechtse Bibliotheek |
Utrecht |
* |
* |
* |
* |
* |
0 |
4 |
12 |
24 |
9 |
24 |
4 |
17 |
32 |
| Netherlands |
Amsterdamse poort |
Haarlem |
* |
* |
* |
* |
18 |
4 |
7 |
3 |
* |
* |
* |
* |
* |
* |
| Netherlands |
Keizer Karelplein |
Nijmegen |
* |
* |
* |
* |
1 |
6 |
2 |
* |
* |
* |
* |
* |
* |
* |
| Netherlands |
Arnhemseweg |
Apeldoorn |
* |
* |
* |
* |
* |
* |
* |
* |
33 |
9 |
20 |
8 |
22 |
17 |
| Netherlands |
Floreslaan |
Vlaardingen |
* |
* |
* |
* |
3 |
5 |
6 |
25 |
13 |
* |
9 |
4 |
15 |
19 |
| Portugal |
Lisboa Bairro Alto - R. do Seculo 51 |
Lisboa |
* |
* |
* |
* |
* |
* |
4 |
6 |
1 |
1 |
2 |
* |
* |
* |
| Portugal |
Montes Chaos |
Sines |
* |
* |
* |
* |
2 |
2 |
13 |
88 |
4 |
0 |
10 |
* |
* |
* |
| Spain |
Plaza Castilla - Avenida Castellana |
Madrid |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
1 |
* |
* |
| Spain |
Poble Nov - Pl.Doctor Trueta |
Barcelona |
* |
* |
* |
* |
* |
30 |
39 |
8 |
1 |
13 |
16 |
* |
* |
* |
| Spain |
Molina Pl. |
Barcelona |
* |
* |
* |
* |
* |
11 |
20 |
16 |
0 |
5 |
9 |
1 |
* |
* |
| Spain |
Montcada I Reixach |
Barcelona |
* |
* |
* |
* |
* |
0 |
0 |
* |
* |
0 |
2 |
* |
* |
* |
| Great Britain |
Central London Lab-Minster House |
Greater London |
0 |
0 |
0 |
15 |
8 |
1 |
0 |
13 |
2 |
* |
* |
* |
* |
* |
| Great Britain |
Bridge Place |
Greater London |
* |
* |
* |
* |
* |
* |
* |
* |
4 |
0 |
0 |
* |
* |
0 |
| Great Britain |
Stevenage - WSL |
Stevenage |
0 |
23 |
31 |
10 |
9 |
4 |
2 |
* |
* |
* |
* |
* |
* |
* |
The data were calculated from all urban sites connected to the AIRBASE database. * : no data
2.5 Photochemical ozone creation potential
Different VOCs have different ozone-generating capacities. The chemical basis for these differences is now reasonably well understood. The concept of the Photochemical Ozone Creation Potential (POCP) is a widely used approach to estimate the relative importance of individual VOCs for the short-term production of O3 (Derwent and Jenkin, 1991; Simpson, 1995). POCP is defined as the change in mean O3 when a particular species is reduced relative to the change in mean O3 when ethene is reduced. The definition of POCP is subject to some discussion because it does not refer to: (1) the transport time scales versus the photochemical reaction time scales, (2) the levels of peroxy radicals and NOx required during the production of O3, and (3) the issue of VOC or NOx limitation. When the intent is to regulate on the basis of O3-forming potentials, rather than on total mass, POCP assessments highlight toluene, ethene, butane and propene as the most efficient short-term ozone producers among the most abundant VOCs. However, if one evaluates POCP values on longer time frames (e.g. 96-h), the slowly reacting alkanes become increasingly important (Andersson-Sköld et al., 1992).
2.6 Source-receptor relations
Source-receptor relations have proven to be a very powerful instrument in the development of abatement strategies, in particular in the case of acidification (Alcamo et al., 1990). In many of the applications, components showing a linear behaviour have been addressed. In the case of acidification, the total deposition over an area can be calculated by summing up all contributions from all relevant sources and species, which can be organised either geographically or by emission sector. In the case of O3 the situation is more complex due to the non-linear relation between the source species VOC and NOx and due to the influence of the background troposphere.
Several workers reported on this issue (Kleinman and Benkovitz, 1987; Stedman and Williams, 1992; Simpson, 1992), however, in much of this work the relations developed were valid under fixed circumstances only e.g. fixed HOx/NOx ratios. Simpson (1992) found that in VOC-limited regimes, ozone shows to a large extent a linear dependence on VOC-emission changes. Source-receptor relations on exceedances of ozone thresholds averaged over longer term periods (3 to 6 months) appeared much more robust than those on the daily maximum concentration, for example (Simpson and Malik, 1996).
The demand for ozone source-receptor relations with a much broader validity range has increased because of the call for cost-effective and spatially differentiated abatement strategy development. The ozone problem will also be linked to acidification and eutrophication in order to achieve optimum emission reductions to meet targets for solving all these problems. Heyes et al. (1996) developed source-receptor relations for ozone in the form of isopleth diagrams, which may serve in the multi-pollutant / multi-effect approach. The work of Heyes et al. (1996) will be applied to support the European Commission in its ozone abatement strategy development (Amann et al., 1997) and the UN-ECE in its work on the second NOx protocol.


Document Actions
Share with others